Discipline email list
Clinical Trials
Guaranteed 95% deliverability
Accurate targeting
Sending service
.csv or excel file delivery
Clinical trials email list
Marketwise’s comprehensive clinical trials email list enables you to market your product or service to specialists engaging in clinical trials around the world. Researchers in our clinical trials email list work in over 50 countries, with the vast majority working in medical research, for clinical research organisations (CROs) and in pharmaceutical company based markets.
Clinical Researchers can be discipline agnostic, therefore it is difficult to associate a particular discipline to them. Marketwise is happy to filter on additional disciplines or research areas to enhance your custom email list for your product or service promotion.
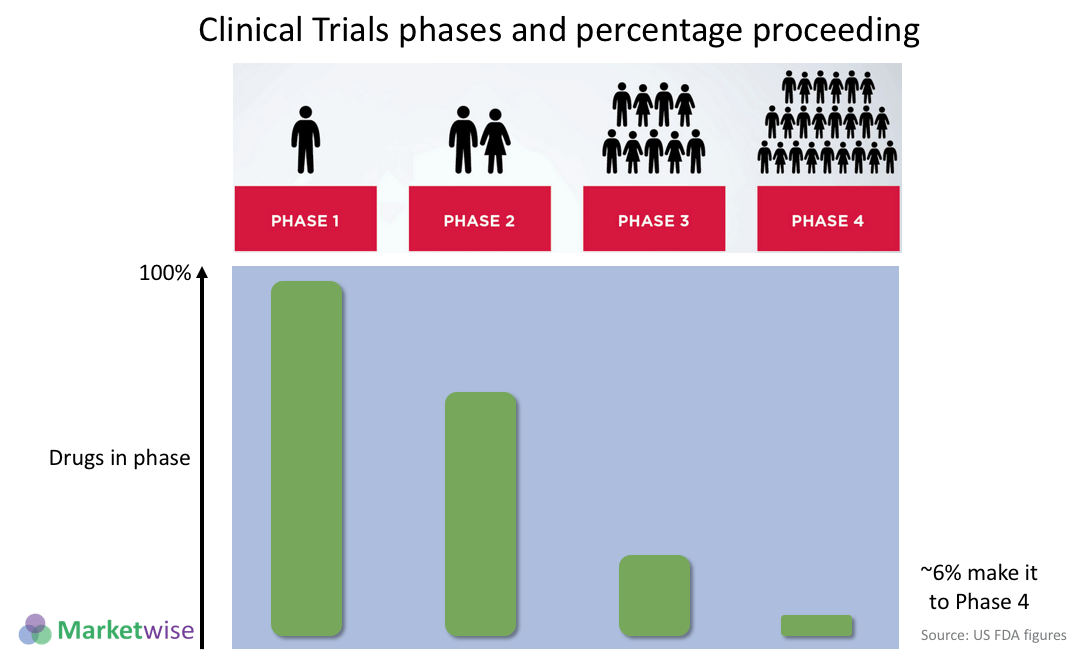
Clinical trials are highly controlled, phased testing. Drug safety and efficacy are critical testing parameter.
Clinical trials are usually performed in four main phases. The use of ‘double blind’ techniques, mean that it is extremely hard for the participants or those running the trial to influence the results. Neither will know if the trial drug or a placebo are being used (as a control). This prevents subconscious bias creeping into the results. Only at the controlled points in the trial, will a supervisor examine the results and confirm which trial participants were actually receiving the drug.
A very high percentage of drugs fail to make it through all the phases, as indicated by the image below:
Accurate data
Marketwise puts resources every week into keeping our data clean, accurate and up to date. We use a combination of both manual effort and automation to ensure our skills, discipline, research areas, job titles and markets are accurate, along with Names. This means your message is more likely to reach the most relevant contact, increasing your chances of success.
Deliverable data
Our multi-stage process means that we are confident in very high (>95%) email deliverability. Our data is always cleaned after every use, with unsubscribes and bounces removed. In other words, you can have confidence your message will actually reach the inbox of the right people.
Learn how you can help deliverability with our guide to avoiding the spam folder.
Geo-targeting
Choose individual countries, regions or combinations, such as "Europe, but exclude Switzerland", or "North America and Brazil".
Data policies compliant
We comply to all relevant laws, such as GDPR and CAN-SPAM so you don't have to worry.
Email count enquiry

Discipline Email List: Pharmacology

Discipline Email List: Pharmacy
Disease Email List: Diabetes

Network Security email list

Blog: Speed v Accuracy email data
FAQ for the Clinical trials specialist email list
Can I customize the Clinical trials marketing list?
Absolutely. Our pre-built Clinical trials specialist email list is easily customized. Choose filters such as the country, other related disciplines or diseases and the market segment of interest. For example:
-
- Clinical trials + Pharmacology + Pharmaceuticals + USA
- Clinical trials specialist + Oncology + Medical Research + Europe and Asia
How do I integrate the Clinical trials specialist data into my CRM?
We make it as simple as possible. Your Clinical trials specialist data is delivered as an industry standard Comma separated value, (CSV) file. When you receive the file from us, save it to a secure location, open your CRM tool and find the “Import Data” or “Import Contacts” option. Upload the Clinical trials specialist CSV file you just saved and the contact data should be quickly imported.
Can you send my Clinical trials specialist email campaign for me?
Yes. We follow the same process to agree your Clinical trials specialist requirements, but instead of delivering the data to you, you deliver your email html file to us. This can be a good option for companies unable to buy email data lists directly. Pricing is available on this page.
What is your process to ensure high quality Clinical trials specialist data?
All our data passes through a highly rigorous classification process. This involves over 500 automated checks for every contact and helps us achieve the highest possible classification accuracy. The second phase to ensuring high quality concerns deliverability. We always clean data using industry standard tools right before delivery to you. Finally, all data is human verified before delivery, since automation still needs a helping hand sometimes. You can be sure your Clinical trials specialist data is accurate AND deliverable.
What is the source of your Clinical trials specialist data?
We only use publicly available information sources of Clinical trials specialists, such as directories and other similar high quality sources. Specifically, we do not engage in web scraping, this results in poor quality data and can land you in trouble with spam regulations.
What payment methods do you accept?
Once you have reviewed and agreed your Clinical trials sample data, we will provide you with an invoice. Payment is either via bank transfer, or credit card (subject to a 5% fee we pass on). Banking details are on the invoice. We accept USD, EUR and GBP. Pricing is available on this page.
What if I am not happy with the results from my Clinical trials mailing list?
If you experience >5% hard bounces from your Clinical trials email list, we will give you replacement data to make up the difference. Unfortunately we can’t guarantee the overall performance of your campaign, which will be greatly impacted by your subject line and email content.
Email list pricing example
Sending a message to 5000 scientists from one of our lists, would cost just $1,330 / €1,110 / £1,000.
That's just $0.27 per message, going directly to the inbox of a highly targeted contact.
Find New, Relevant Sales and Marketing Leads for Your Teams
Product and Service Promotion
New Lead
Generation
Fill your sales pipeline with potential new leads. Push your marketing message out to new contacts in the Biotech and other key areas.
Build new interest and start to create your own mailing list with converted contacts.
Event
Promotion
Grow your attendees for your cardiology and other science focused webinars with early promotional campaigns to encourage registration.
Use our dedicated conference package to send a sequence of messages to targeted scientists.
Example organisations in the Clinical trials email list
- Parexel International Corp
- Novartis
- GlaxoSmithKline
- inVentiv Health
- Covance Inc
Example contacts in our Clinical Trials email list
- Scientist, Clinical Research, PAREXEL International Corporation, (in multiple countries).
- Head of Department, Global Clinical Research and Development, GlaxoSmithKline, Belgium
- Manager, Clinical Research, Novartis, New Brunswick, Canada
- Researcher, Phase I Clinical Research Unit, Shanghai Xuhui Central Hospital, Shanghai, 200031, China
- Scientist, Alzheimer’s Disease Clinical Research Centre, Gérontopôle, Toulouse University HospitalINSERM 1027, France
- Researcher, Department of Small Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI, USA
Life science
email lists
Medical science
email lists
- Anatomy
- Bacteriology
- Cardiology
- Clinical Chemistry
- Cytogenetics
- Dental Science
- Dermatology
- Endocrinology
- Epidemiology
- Hematology
- Immunology
- Infectious disease
- Medicine
- Neurology
- Nutrition
- Obstetrics and Gynecology
- Oncology
- Ophthalmology
- Paediatrics
- Parasitology
- Pathology
- Pharmacology
- Pharmacy
- Pulmonology
- Rheumatology
- Urology
Applied life science
email lists
Other life science
email lists
Physical science
email lists
Get in touch and tell us what you need !
Email list count enquiry form
Please get in touch and let us know what you need. Useful information includes any custom searches or what roles, markets and skills are of interest. Any extra information you can provide will help us create the best email list for your requirement.
We look forward to hearing from you.